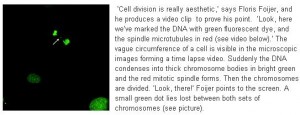
Chromosomes, ageing and cancer
In around two thirds of all cancers there is chromosome instability: during cell division the chromosomes are not distributed equally between daughter cells. But chromosome instability does not in itself cause cancer. ERIBA group leader Floris Foijer tries to elucidate the link between chromosome instability, ageing and cancer.
‘There’s a chromosome that got lost and ended up in the wrong cell. That is how chromosome instability results in aneuploidy.’ Aneuploidy means that cells have the wrong number of chromosomes. A well-known form of aneuploidy is Down Syndrome, where patients have an extra copy of chromosome 21 in all of their cells.
Trained as a biotechnologist and process engineer at Wageningen University, Foijer developed a keen interest in medical research. This led to a PhD project at the Netherlands Cancer Institute NKI in Amsterdam. ‘There I discovered a control mechanism, a sort of emergency brake to stop cell division. But the mechanism came at a price: when these cells started dividing again, they were susceptible to chromosome instability, a failure to maintain the correct number of chromosomes.’
Aneuploidy probably predisposes cancer
Two thirds of cancer cells show chromosome instability resulting in aneuploidy. ‘Aneuploidy probably predisposes cells to cancer. But not all cells with the wrong number of chromosomes become cancerous. You need something in addition, such as a mutation that shuts down the cancer suppressor gene p53.’ After moving to ERIBA in 2011, Foijer continued to work on the role played by chromosome instability in cancer and in ageing. He set out to make a mouse model in which he could study the effects of instability under controlled circumstances. The effort was a success, and led to a unique mouse model. ‘We can now create mice that have chromosome instability in specific tissues.’
Last year he produced the first results, using mice with chromosome instability in the skin. ‘An interesting observation is that the stem cells in hair follicles died off, while the skin stem cells survived. But the skin showed dramatic aneuploidy.’ Apparently, hair follicle stem cells cannot handle chromosome instability and perish, while the skin cells survive. ‘The interesting question for me is: would it be possible to make cancer cells behave like those hair follicle stem cells, so that the chromosome instability will kill them? But to get there, we need to understand what is happening in different tissues.’
Metabolic stress as a marker of cancerous cells
Another finding is that aneuploidy causes cells to use more energy because more genes are active. That would seem logical: if a cell has an extra chromosome, it produces extra copies of many genes on that chromosome. ‘But you would expect the cancer cells to do something about this, such as shutting down those superfluous genes,’ says Foijer. ‘But they don’t.’ The extra energy they put into the activity of these extra genes causes metabolic stress: energy production is driven into the red. ‘That makes the cells really queasy. And this stress is a marker of cancerous cells, so it is a possible target for anti-cancer therapies.
But what does all of this have to do with ageing, the central theme of the ERIBA institute? Foijer smiles. ‘A good question. First, the chance of getting cancer increases with age. But more directly, in the aneuploid skin of our mouse model we saw no cancer but we did see increased ageing. A collaborator of ours noticed the same in a different model.’ Possibly, aneuploidy causes rapid ageing, which eventually causes cancer. ‘Control mechanisms that prevent aneuploidy will cost the cell energy. So maybe fast dividing cells have sloppier controls, which would be acceptable for skin cells that last only three days.’
Prognostic value?
Foijer would be interested to know whether there is a greater degree of aneuploidy in the skin of old people. ‘We could answer this using the LifeLines biobank, which stores biological material and health information relating to 165,000 people in the Dutch northern provinces. Perhaps aneuploidy could have a prognostic value for cancer or ageing.’
Having access to LifeLines, which was launched by the UMCG University Hospital, is one of the advantages of working at ERIBA. ‘But also the fact that I can collaborate with clinicians in, for example, oncology or pathology. And, of course, the collaborations inside ERIBA. I find that all ERIBA Group Leaders have some link with my own research, even though they work on different subjects with different model organisms. That truly stimulates collaboration.’ And all that is paying off. ‘I’ve obtained some wonderful results. There are a few interesting papers in the pipeline.’
Text: René Fransen
Back to previous page